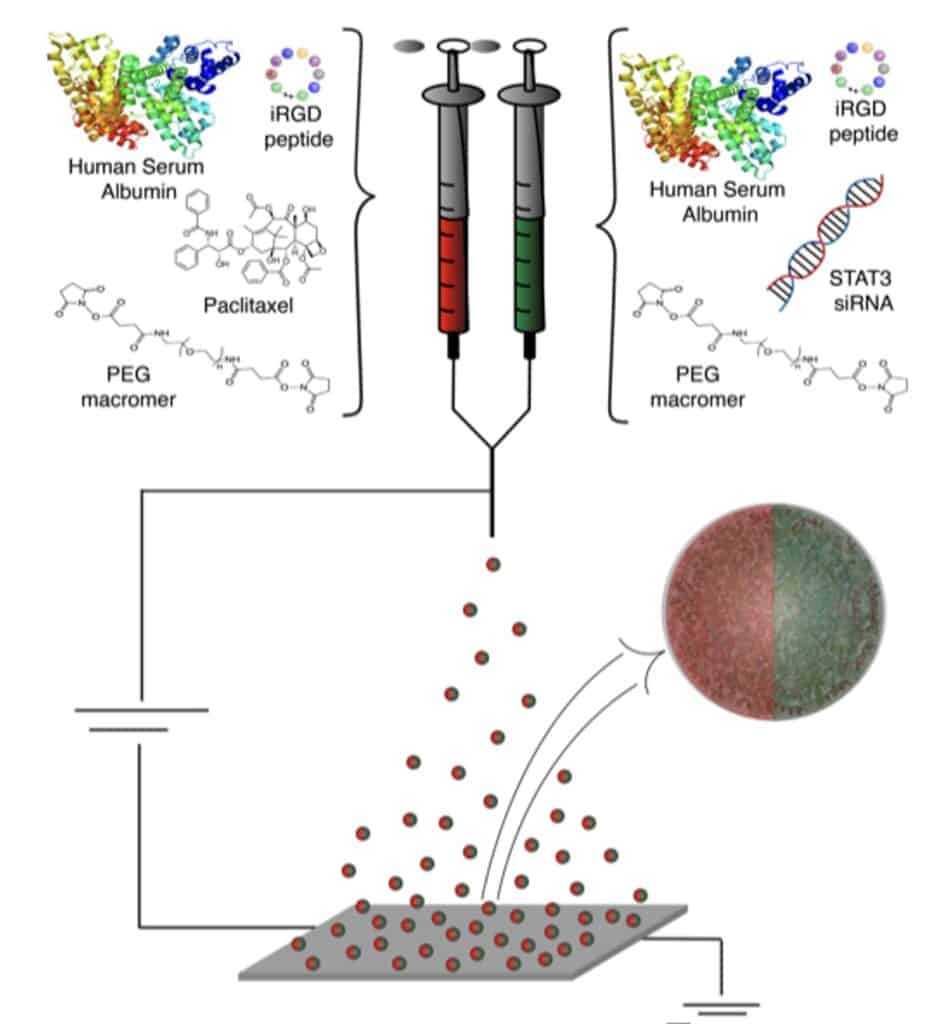
An extended diagram shows the components that go into each liquid—the human serum albumin that helps cross the blood-brain barrier, the iRGD peptide that helps to target the tumor and the PEG macromer, a biodegradable polymer that holds it all together, plus the tumor-killing drug Paclitaxel or the immune activator STAT3 siRNA. Credit: Jason Gregory, Lahann Lab, University of Michigan.
A new nanomedicine out of the University of Michigan uses a protein that crosses the blood-brain barrier carrying a drug that kills tumor cells and another drug that activates the immune system to fight brain cancer. That's the news along with an announcement of $2.38 million to test the new cancer treatment in mice in Ann Arbor. The new medicine is targeting the most aggressive form of brain cancer, glioblastoma. The funding comes from the National Institutes of Health to test the technology at the University of Michigan.
The research will be led by a nano-engineer and neuro-oncology researchers at U-M, and is the first study to test the two drugs together in a way that can be delivered through the bloodstream rather than a hole in the skull. This research builds on previously successful work eliminating cancer in seven of eight mice by packaging only the immune drug in the protein that crosses the blood-brain barrier so that it could be delivered intravenously.
The five-year survival rate for glioblastoma in humans is 5 percent, a dismal statistic. "The standard of care for glioblastoma is surgery and radiation, and the median survival hasn't improved for several decades. A systemically delivered nanomedicine that can prolong survival and prevent recurrence is the dream," said Maria Castro, the R.C. Schneider Collegiate Professor of Neurosurgery and professor of cell and developmental biology. Her team leads the mouse studies in collaboration with Pedro Lowenstein, the Richard C. Schneider Collegiate Professor of Neurosurgery and professor of cell and developmental biology.
As the team tests out the nanoparticles timed to release the immune drug followed by a tumor-killing drug, developed and produced by project lead Joerg Lahann's group, one of the primary questions will be how well the drugs work together. "Are they working much better than either drug alone? That’s what we’re hoping for. Or is it just a small improvement—or are they actually competing with each other and making the treatment worse or increasing the side effects?" said Lahann, the Wolfgang Pauli Collegiate Professor of Engineering and director of the U-M Biointerfaces Institute.
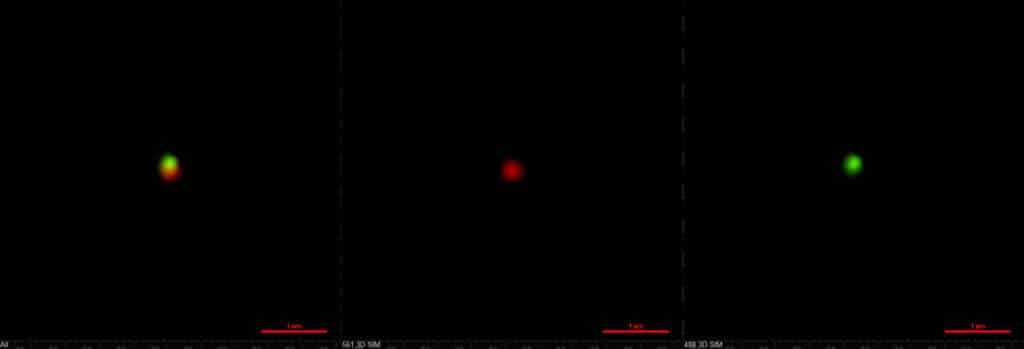
The two-compartment nanoparticles as seen with structured illumination microscopy. The green compartment contains the immune drug while the red compartment brings the tumor-killer. Credit: Ava Mauser and Nahal Habibi, Lahann Lab, University of Michigan.
The advanced nanomedicines will be delivered intravenously and combined with radiation therapy, as they will be in a future clinical trial for humans. Lahann's team packages the drugs in a protein called human serum albumin, which is present in human blood, to get the drugs from the bloodstream into the brain. Once there, the drugs attempt to wake up the immune system to prevent recurrence of the cancer and death, which is a common occurrence with this particular type of cancer following surgery or chemotherapy.
Tumors can regrow because cancer cells have ways of suppressing the immune system, the researchers say. The 2020 study and this new grant use a drug that blocks STAT3, a signaling molecule that cancer cells use to tell immune cells not to attack them. This allows the immune system of the mice in the study the ability to identify cancer cells as targets for destruction.
In a study published in May, the team used a drug that blocks CXCR4, an immune receptor that receives orders to send killer T-cells away. Blocking CXCR4 helps keep T-cells in the brain, where they do their work of killing brain cancer cells. Three out of five mice from the study survived long term, and all of those survivors cleared new tumors during the recurrence challenge, remarkable progress for a difficult disease.
The new grant won't use this drug, but the team is interested in a future study exploring whether two different immune approaches together might be even more effective.
"Tumors have a lot of variation, so we need to attack them from many directions," Lowenstein said.
Previous studies suggest that the nanoparticles home in on tumor cells, infiltrating them more often than healthy cells. One of the goals for this study is to better understand how that works. "For nanomedicines to advance into clinical trials as experimental treatments for glioblastoma, we must understand the mechanisms by which they accumulate in tumor and other tissues," said Colin Greineder, U-M assistant professor of emergency medicine, who will lead studies of how the nanomedicine distributes in the body.